Number of Electrons to Fill Outer Shell of Sulfur
1 The electronic configuration of carbon is 1s2 2s2 2p2. Sulfur has 6 outer-shell electrons this is because it.
Atoms Isotopes Ions And Molecules Boundless Biology
So Sulfur with electron configuration of 286 in its outer valance shell will need to GAIN two electrons to get 8 and have a stable outer shell.

. The electron configuration of sulfurS shows that there are two electrons in the K shell eight in the L shell and six in the M shell. K shell n 1 2 electrons. That means there are 16 electrons in a sulfur atom.
This will mean there are 2 more electrons then protons and the overall ion will have a negative charge. Why the number of elements in third period is 8. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital.
Sulfurs outermost shell is 3p. The octet rule is based on the fact that each valence orbital typically one ns and three np orbitals can accommodate only two electrons. What is the number of electrons to fill outer shell of sulfur.
Thus n1 shell can hold two electrons. Shell 2 8. In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom there are 16 electrons.
1 number of electrons needed to fill outer shell. 42 Votes Each shell can contain only a fixed number of electrons. Out of 6 possible electrons in the 3p orbital only 4 electrons fill the shell.
415 1045 Views. B The number of orbitals with the quantum numbers n 312 and ml c The number of valence electrons in the outermost p sub-shell of a Sulfur atom d The number of unpaired electrons in a Mn2 ion e The ml values allowed for a. The first shell can hold up to two electrons the second shell can hold up to eight 2 6.
The shell closest to the nucleus 1n can hold two electrons while the next shell 2n can hold eight and the third shell 3n can hold up to eighteenThe number of electrons in the outermost shell of a particular atom determines its reactivity or tendency to form chemical bonds with other atoms. The next energy level the last one is the outermost energy which comprises. But the last shell cannot accommodate more than 8 electrons so the number of electrons in.
The number of valence electrons needed to fill its shell can be determined by inputting the shell level n squaring it and by multiplying by 2. How do electrons fill in shells. An s-orbital holds 2 electrons.
Outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. When we write the configuration well put all 16 electrons in orbitals around the nucleus of the Sulfur atom. Carbon atoms with four electrons in the outer shell need four more electrons to reach a stable electron configuration such as a noble gas.
Sulfur has two electrons in the 1s orbital two electrons in the 2s orbital and six electrons in the 2p orbitals. The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on. In this configuration we note that there is only one electron in the 3rd energy level.
From this we can see that the carbon atom has four unpaired electrons which are in the outer shell called the valence electron. Atoms prefer to gain the stability of octet by having eight electrons in the outer shell the electrons of the s and p orbitals. These electrons are part of the first and second energy levels the electron core.
If you only want to find valance electron in sulphur atom there is a very simple answer for you. So for the element of SULFUR you already know that the atomic number tells you the number of electrons. Sulfur has 6 outer-shell electrons this is because it is in group 6A of the periodic table.
How many electrons are needed to fill the outer shell of Sulfur. Therefore sulfur has 4 valence electrons. Each shell can contain only a fixed number of electrons.
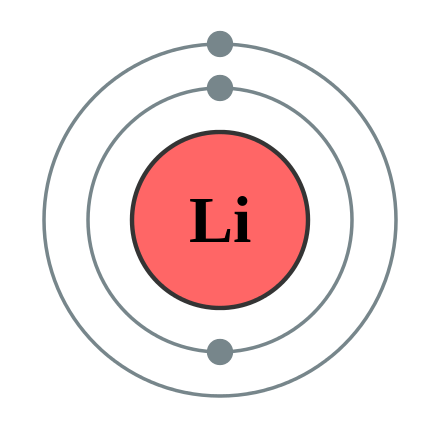
S-orbitals can hold 2 electrons the p-orbitals can hold 6 electrons. The n2 second shell has. Because lithiums final electron goes into the 2s subshell we write the electron configuration of a lithium atom as 1s 2 2s 1.
By bonding with two hydrogen atoms oxygen has its 6 outer shell electrons 2 shared electrons from the hydrogen making 8 and a filled shell makes a happy atom. How many full electron levels does sulfur have. We know that sulfur atoms have a total of sixteen electrons.
12 marks Fill in the blanks. A The ground state electron configuration Kr5s4d95p is for the element. Looking at the picture you can see there are two electrons in shell one eight in shell two and six in shell three.
L shell n 2 4 electrons. Shell 1 holds 2 valence e. If an atom of sulfur atomic number 16 were allowed to react with atoms of hydrogen atomic number 1 which of the molecules below would be formed.
The number of valence electrons needed to fill its shell can be determined by inputting the shell level n squaring it and by multiplying by 2. Of sulphur is 16 hence its electronic configuration is 286 1s22s22p63s23p4 as it has 6 electrons in its outermost shell so it has six valance electrons. Because lithiums final electron goes into the 2s subshell we write the electron configuration of a lithium atom as 1s 2 2s 1.
According to the 2n2 rule the maximum number of electrons in the third period 2 x 32 18. Sulfur has an Ne3s23p43d0 electron configuration so in principle it could accommodate more than eight valence electrons by using one or more d orbitals. How many total shells can an atom have.
Hydrogen number of electrons in outer shell. Thus to find the number of electrons possible per shell. Therefore sulfur has 6 valence electrons 25-Jul-2020.
That is the first shell of sulfur has two electrons the second shell has eight electrons and the 3rd shell has six electrons. First we look at the n1 shell the first shell. Since 1s can only hold two electrons the next 2.

Electron Dot Diagram Worksheet Worksheets For School Newpcairport Chemistry Worksheets Worksheets Word Problem Worksheets

12 Mg Magnesium Electron Shell Structure Schoolmykids Element Chemistry Magnesium Electron Configuration
Chem4kids Com Sulfur Orbital And Bonding Info

Sulfur Valence Electrons How To Discuss

Webelements Periodic Table Tungsten Properties Of Free Atoms

Electron Shells Elements 1 18 Youtube
Chem4kids Com Sulfur Orbital And Bonding Info
![]()
Electron Shell Diagrams Of The 118 Elements
Chem4kids Com Sulfur Orbital And Bonding Info

Electron Shell Diagrams Of The 118 Elements

How Many Electrons Can The Third Energy Level Hold At Level
Electron Configuration Wikiwand
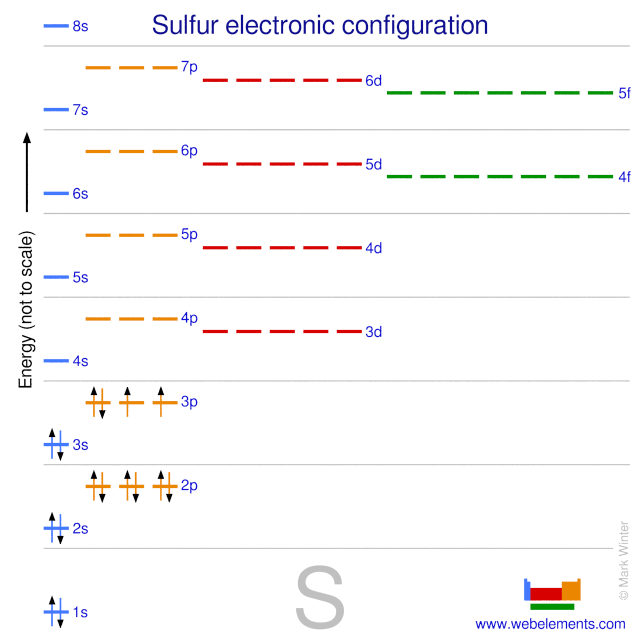
Webelements Periodic Table Sulfur Properties Of Free Atoms
How Many Electrons Can The Third Energy Level Hold At Level

Question Video Identifying The Number Of Electrons In The Outermost Electron Shell Of An Atom Nagwa
Chem4kids Com Selenium Orbital And Bonding Info

Webelements Periodic Table Sulfur Properties Of Free Atoms

Chemistry Card Game Printable Electron Match Learning Game Etsy Card Games Activity Cards Chemical Bond
Comments
Post a Comment